Figure 3
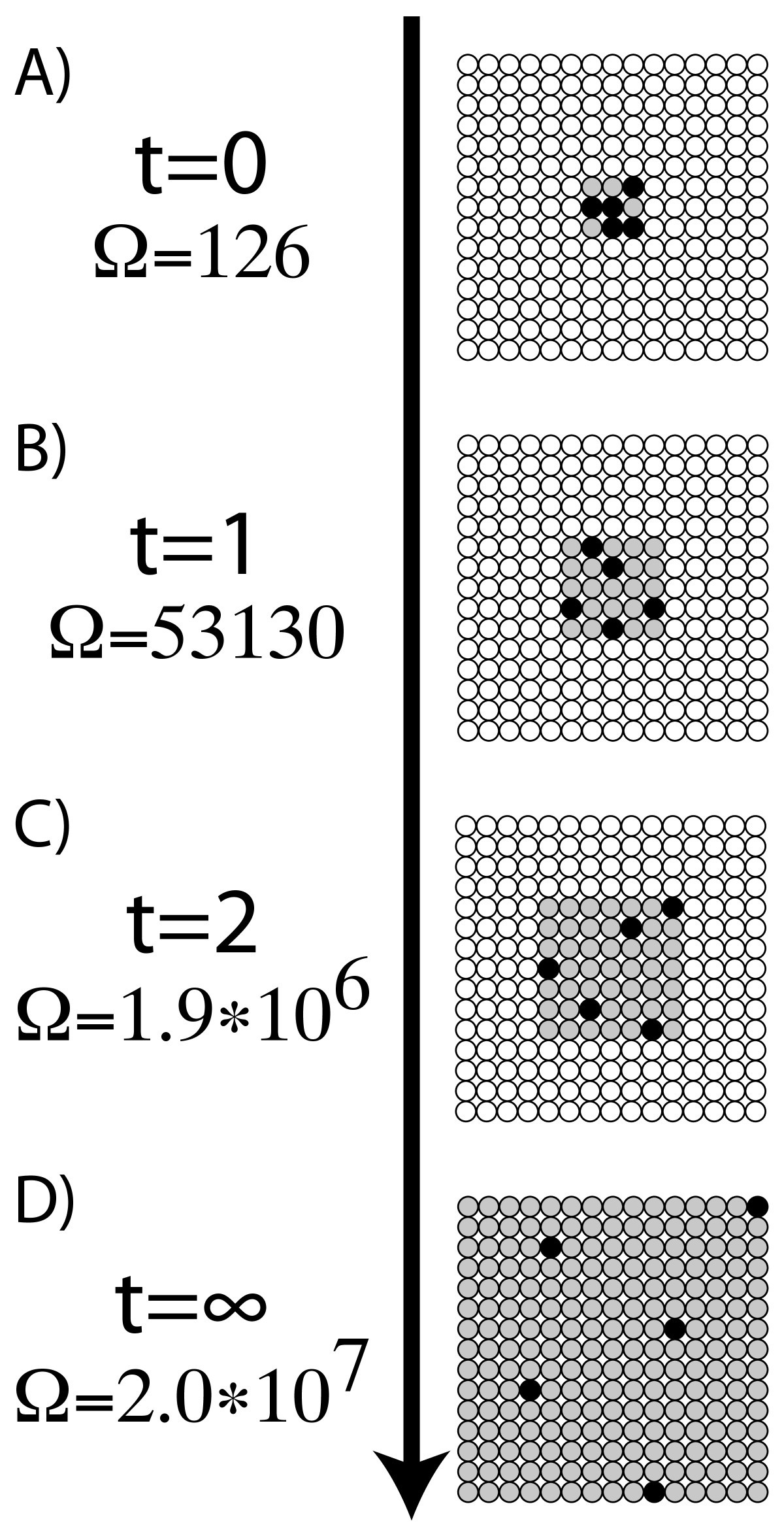
A microscopic example of the link between multiplicity and entropy consisting of the simplified system of diffusing dye molecules. (A) When the five molecules of dye (black circles) are introduced to the solution, they can only inhabit a very small area (grey circles). This limits how many ways the dye molecules can be arranged, resulting in a lower multiplicity and lower entropy. As time progresses, the dye molecules have access to more spaces in solution. (B) At time t = 1, the possible number of spaces the dye can inhabit is 25, resulting in a multiplicity of 53,130. (C) The next time step (t = 2), increases the accessible spaces even more. (D) Once the dye molecules have diffused and are able to inhabit all the spaces, the solution has reached a maximum multiplicity and entropy.